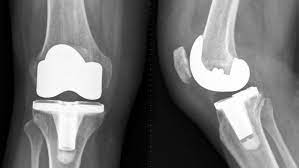
The remember initial grew to be general public in February 2022 when Exactech given an Emergency Medical Product Correction Notice educating doctors that the majority of the Exactech knee inserts that have been produced from 2004 until 2022, comprised nonconforming packaging tiers about the extra-higher-molecular-bodyweight polyethylene (UHMWPE) elements. Especially, the packing levels for that plastic material insert let a lot of fresh air to diffuse into the insert even though it is simply being placed and before it is actually implanted, which can cause an operation referred to as oxidation.
As part of the same February 2022 recognize, Exactech recalled its total leg replacing devices that have been manufactured involving the many years of 2017 and 2022. Just like the joint recall, the polyethylene (plastic-type) put that suit involving the tibial element and the talar aspect as being the new cushion or cartilage for that changed ankle joints, covered exactly the same defect that enabled the plastic material to get oxidized, which may result in the plastic material to wear out prematurely or become broken after it really is implanted to the patient’s physique.
Exactech Knee Recall Lawsuit are the pursuing Exactech leg and ankle solutions:
•Optetrak: 60,926 implanted devices since 2004
•Optetrak Logic: 60,518 implanted models since 2004
•Truliant Joint Substitute: 24,727 inserted units since 2004
•Vantage Ankle joint Implants: 1,561 inserted considering that 2004
Exactech also recalled around 90,000 hip substitutes with Exactech Connexion GXL Liners in June 2021, since the plastic material is made employing a “moderate” go across-linking method, which is inherently a lot more susceptible to oxidation and rapid wear leading to bone fragments reduction/osteolysis. On August 11, 2022, this recall was broadened after Exactech discovered a similar deficiency in the packaging in the plastic material liners resulting in greater oxidation in the plastic tiers, and ultimately for the patient, resulting in increased put on and bone loss and component low energy cracking/fracturing. This additional recall widened the recalled stylish gadgets from 2015 back to since 2004, delivering the entire number of Exactech recalled stylish gadgets to approximately 125,000.
As a result of these defects, some sufferers have required revision surgical procedure to take out the been unsuccessful plastic-type material put along with other components of these products. Deterioration in the polyethylene by yourself, and potentially together with any other style problems, results in aspect loosening, muscle damage, osteolysis, long lasting bone tissue reduction, and also other accidents, resulting in intricate revision surgical procedures and substantial recovery time.
However, Exactech has not yet yet directly alerted individuals that their items are recalled, but alternatively are depending on doctors to know their patients whether they are impacted by the malfunctioning units.